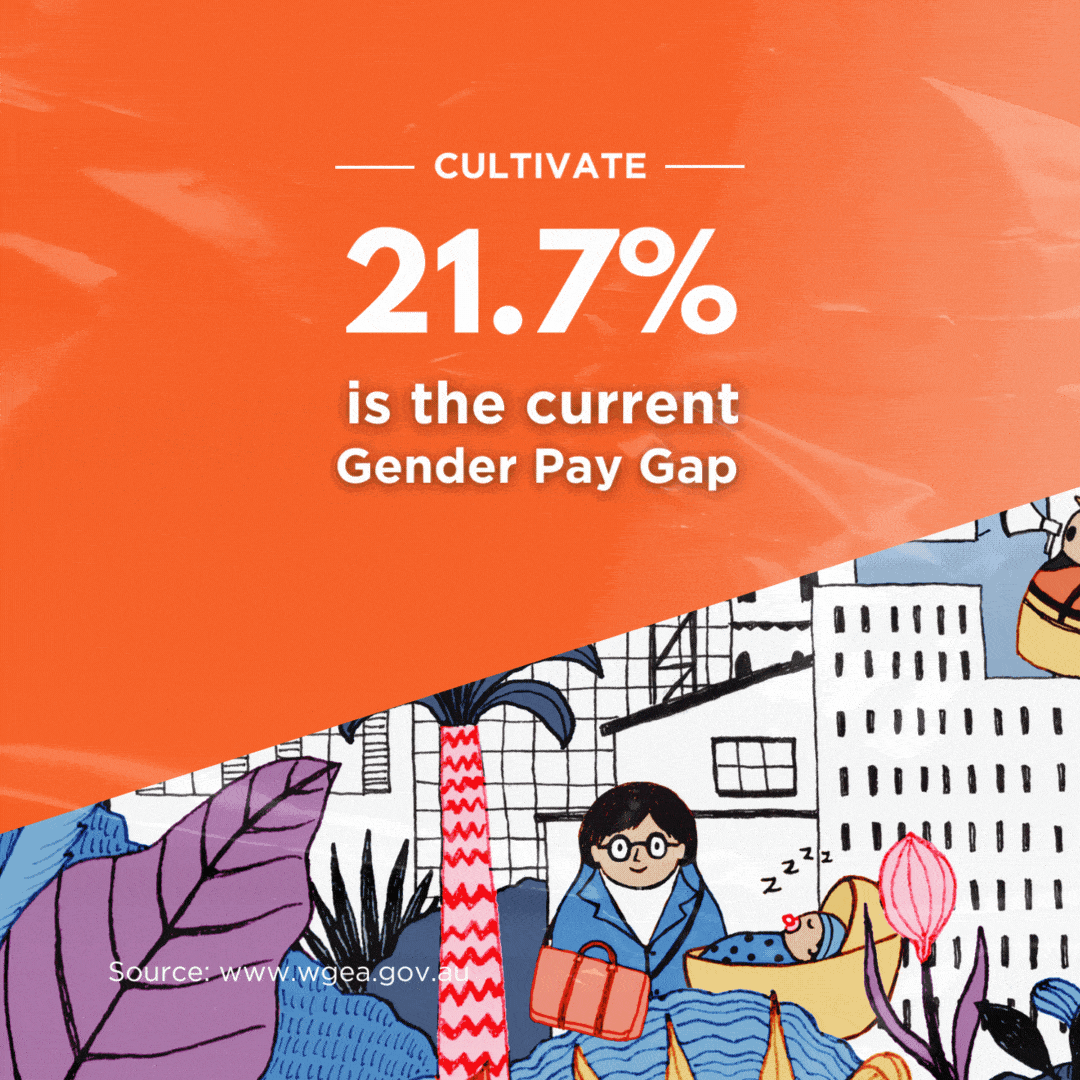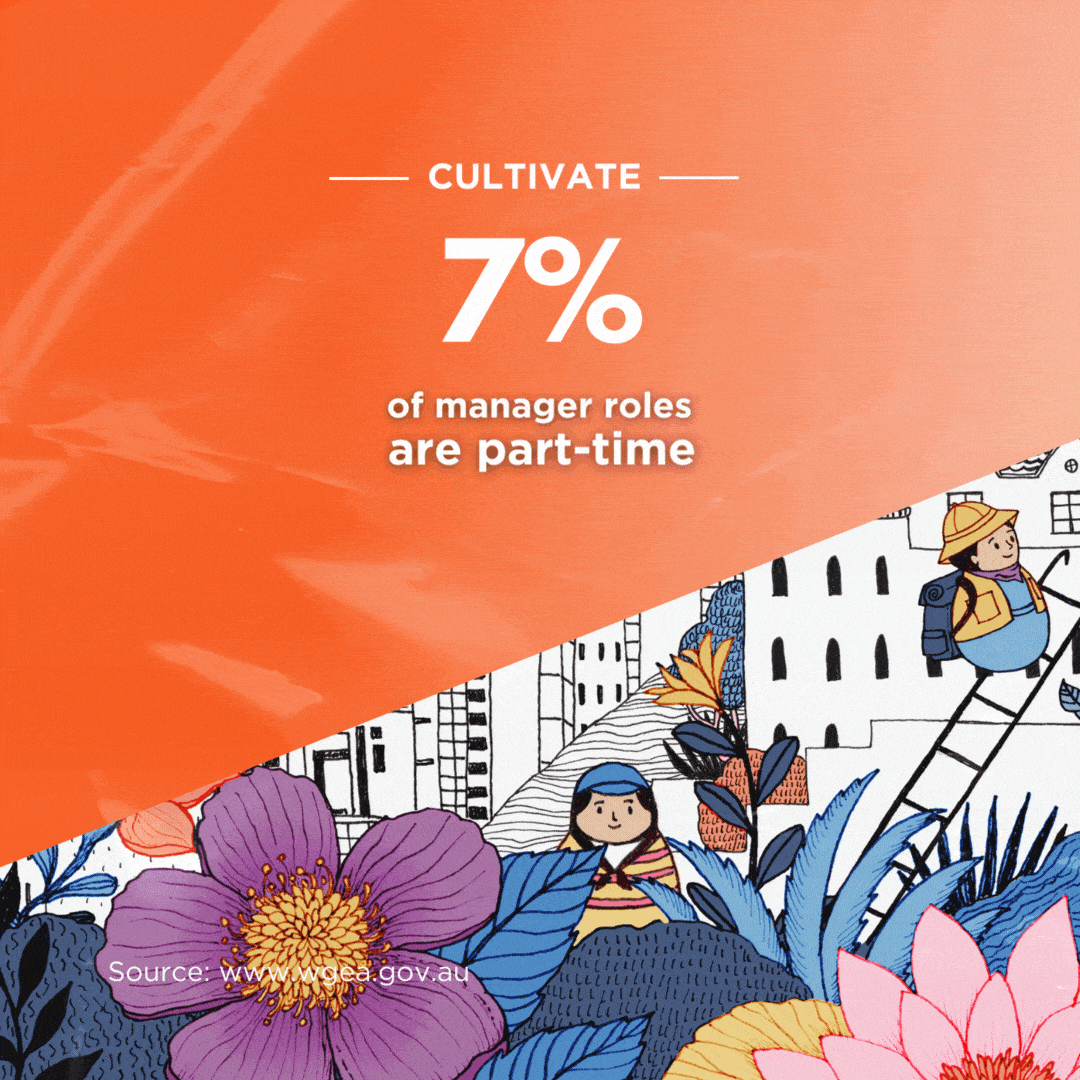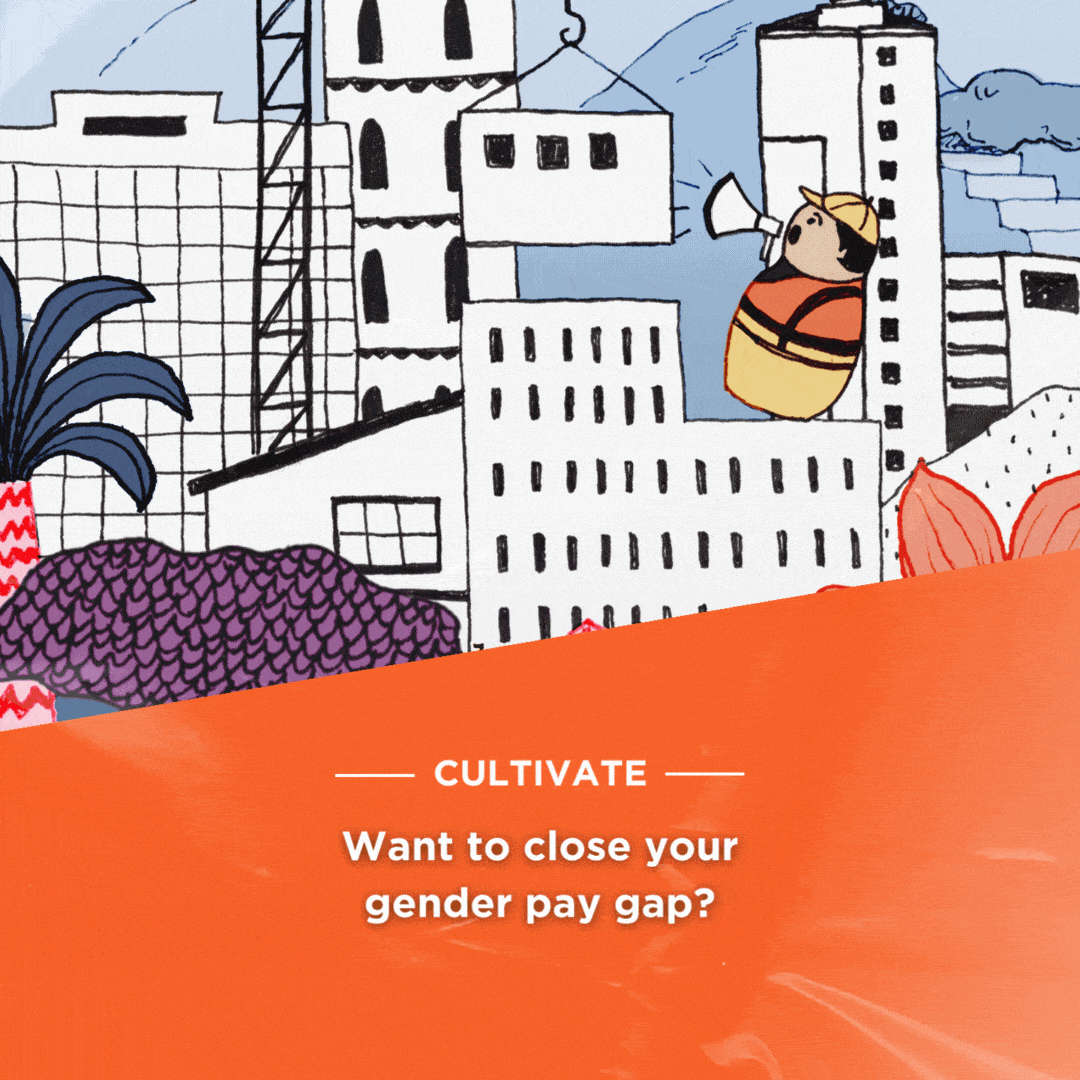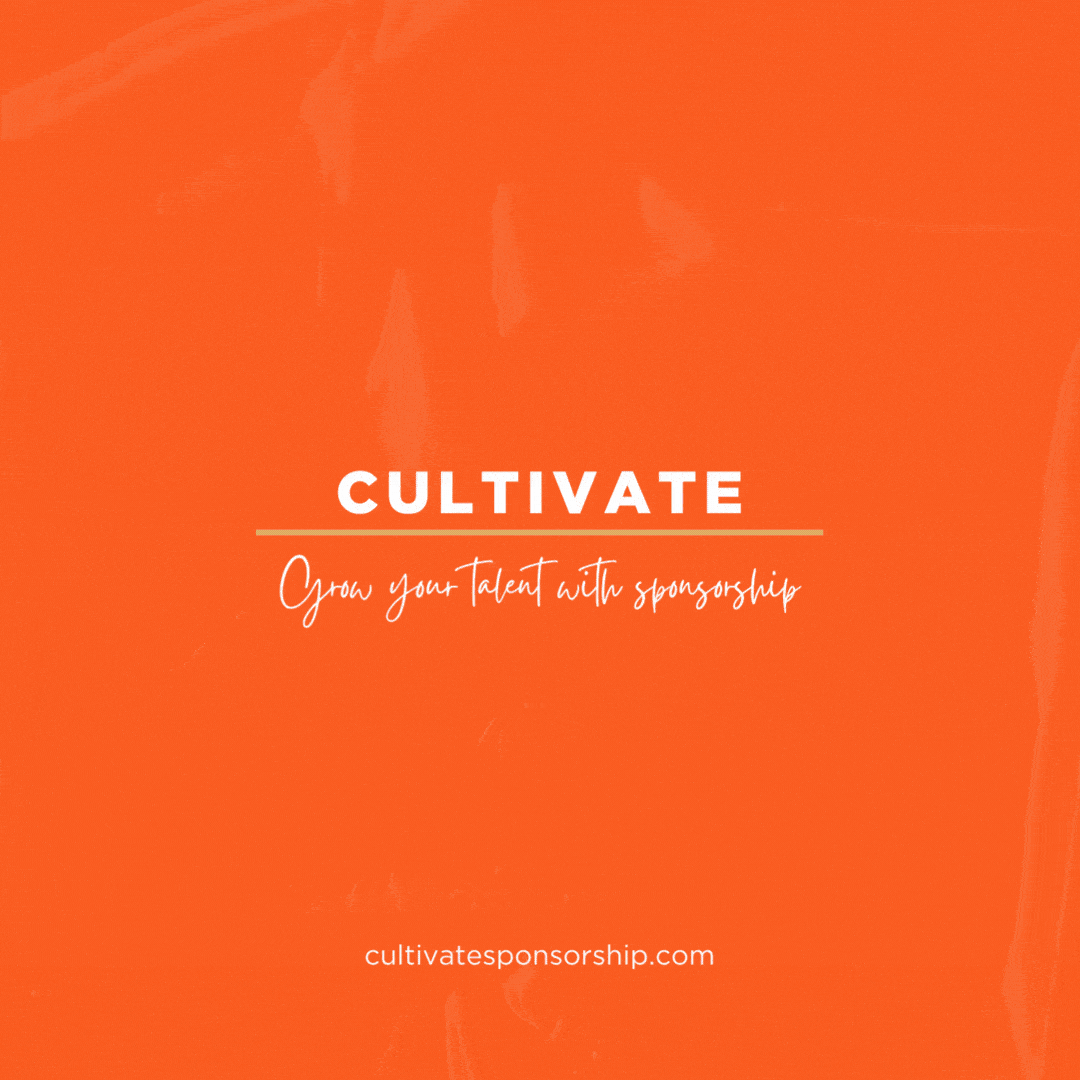
Britain will allow mass immunisations against COVID-19 within weeks after it granted emergency authorisation on Wednesday to Pfizer’s coronavirus vaccine. The Pfizer/BioNTec dose had been cleared for use by the Medicines and Healthcare products Regulatory Agency (MHRA) – the UK’s medical regulator.
The approval process was headed by a woman. But there’s a lot more great women in STEM involved across the full ecosystem of vaccine distribution (just as there has been on vaccine development all over the world).
June Raine is joining the ranks of women in the UK charging ahead with the aim of distributing vaccines, including Chairwoman of the UK Vaccine Taskforce, Kate Bingham and Christina Dold from the Oxford Vaccine Group.
And the organisation behind the vaccine itself, BioNTech which teamed up with Pfizer, is led by a husband and wife team, including Dr Özlem Türeci. She is the company’s Chief Medical Officer, described as a “German physician, immunologist and businessperson of Turkish origin. The couple (now billionaries) married in 2001, and are said to have left their lab only briefly to go to the registry office. They had a daughter four years later.
June Raine told media today that they have beenb able to “authorise the supply of this vaccine using provisions under European law which exist until 1 January,” Raine said. MHRA’s goals are to assess the vaccines that are supposed to end the coronavirus crisis, and ensure that the rush to get them into action does not come at the cost of patient safety. Recently, Raine remarked at a press conference that: “The safety of the public will always come first.”
“There is absolutely no chance that we will compromise on standards of safety or effectiveness,” she said. “Our speed, or our progress, has been totally dependent on the availability of data in our rolling review and the rigorous assessment and independent advice we have received. So I hope that clarifies the point about the European relationship.”
The approval has faced some backlash, with some people believing the UK’s speedy decision was for political incentives, and that they acted beyond European laws. Raine said the procedure was achieved under the terms of European law and stressed that regulators were working under the terms of EU law.
In the recent past, the MHRA has faced criticism for being “a black box, an organisation that hasn’t always followed the highest standards of transparent, reproducible science – they do the basics well, but if you don’t do things openly you can put public trust at risk” according to one expert.
Raine became director of the MHRA’s vigilance and risk management of medicines division in 2006 and has since tightened dosing recommendations for children’s paracetamol, including the popular liquid paracetamol Calpol. She has also introduced new warnings on addictive opioid medicines and removed co-proxamol from sale after it was linked to suicides which has saved hundreds of lives.
Susan Cole, an advocate from the Valproate Stakeholders Network, told The Guardian that Raine was “a trusted figure for families with children who were harmed by the epilepsy drug sodium valproate when she chaired public hearings into its use that ultimately led to the curtailment of its use during pregnancy.”
“She handled the top people from the royal colleges and the NHS, she made sure they heard us. And she made sure they heard her,” she said. “With us, she stood up to people. She was measured, understanding; she was a public servant, most of all. There aren’t that many of them left.”
Regulators in the UK rely on companies’ own analyses, assessing reports published by vaccine makers and basing their decisions on these results. British health secretary, Matt Hancock, declared on Wednesday “Help is on its way with this vaccine — and we can now say that with certainty, rather than with all the caveats.”
On Times Radio, Hancock acknowledged the work of the MRHA in helping the UK became the first country in the world to clinically authorize a vaccine.
“…the MRHA has done a great job of working with the company to look at that data as it’s come through and do things in parallel, rather than one after the other as they normally would,” he said. The latest approval in the UK unfortunately will not impact the distribution of the hundreds of millions of doses that other wealthy countries have aquired in prepaid contracts.
How it will work
The Pfizer vaccine is a joint effort between the American pharmaceutical giant Pfizer, which is one of the world’s largest biopharmaceutical companies, and the German firm BioNTech.
The vaccine was 95 percent effective in a late-stage clinical trial and caused no serious side effects. The distribution process is complicated and challenging; for instance, the vaccine must be stored at roughly minus 70 degrees Celsius until shortly before it is injected. It must be transported in boxes stuffed with dry ice and as soon as it’s removed from the fridge it needs to be used within six hours. Each person needs two doses, one month apart — for the treatment to work.
Though the vaccine can be storied in a normal refrigerator for five days, the government expressed concerns that the logistics involved in moving, defrosting and preparing the vaccine will restrict distribution to only within hospitals for now. Hospitals in the UK will begin vaccinating people over the age of 80 who already have doctors’ appointments booked for the coming weeks. Nursing-home workers and doctors and nurses will also be among the first to be vaccinated.
Nursing homes, smaller clinics and doctors’ offices will follow after. 800,000 doses of the vaccine will be available by next week as the military are getting involved in distributing them. The UK has pre-ordered 40 million doses and is part of a range of different vaccines that the government has ordered.
Manufacturers will now be tasked with needing to produce hundreds of millions of doses of the vaccine before shipping them to hospitals, clinics and pharmacies without compromising their delicate composition.
The chemistry underlying Pfizer’s vaccine had never before produced an approved shot, though the same class of vaccines has long been tested for other uses. In order to coax cells to make a viral protein, called spike, and elicit an immune response, the vaccine delivers genetic instructions, known as messenger RNA, encased in tiny fat globules.
Dr. Ugur Sahin, the chief executive and co-founder of BioNTech, told the NYTimes that the UK’s approval, “will mark the first time citizens outside of the trials will have the opportunity to be immunised against Covid-19.”
Pfizer says it expects to produce 50 million doses by the end of 2020. They plan to ship half of them the US. They also hope to provide the vaccine to up to 25 million people around the world before 2021. In the UK, the virus has so far killed nearly 70,000 people.
Photo Credit: EPA