Funding from the U.S. National Institutes of Health is going to Lisa Jackson as lead researcher into a clinical trial of an investigational vaccine for the coronavirus.
The Kaiser Permanente Washington Health Research Institute is recruiting people for the study led by Jackson, a senior investigator at the Institute. Jackson has already started recruiting dozens of volunteers in Seattle to be the first people to receive the vaccine to test its safety.
Jackson told NPR last week that even if the virus completely goes away, “the phenomena that led to this virus is going to happen again as this nexus of animals and humans and the jump in certain viruses that have circulated in animals into humans, so we need to be more prepared.”
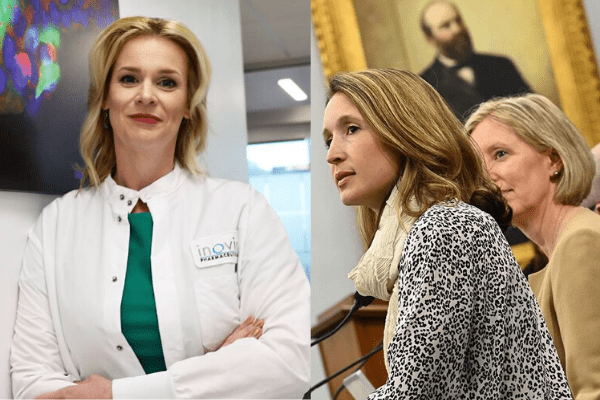
Also in the U.S, Dr Kate Broderick, senior vice president of Inovio Pharmaceuticals, a biotech company that creates immunotherapy technologies to treat and prevent infectious diseases and cancer, is also leading the charge for developing a vaccine.
On January 11, the San Diego-based laboratory received a genetic sequence of the coronavirus from the Chinese government. Her lab took three hours to design a DNA-based medicine against the virus, then synthesised the piece of DNA in coding the vaccine, before putting it into manufacturing.
In a televised broadcast interview, Dr Broderick said “In the past two months have started testing that vaccine in the laboratory and we’ve been very excited by the results that we’ve generated thus far.”
Broderick hopes the results will be used to take the vaccine into human clinical testing in next month.
She told the UK Courier that although the first clinical trials are just weeks away, the vaccine will have to pass several phases of testing before it can be rolled out to the public and that it was “difficult to say” when that would happen.
“Once we’ve completed phase one we go to the regulatory authority and ask to start phase two, a much larger trial involving potentially thousands of people,” she said. “At that stage we would really focus in on people like healthcare workers and those with a high chance of actually coming across the virus.”
“We really hope to start that by the end of the year but there is a lot to do before we reach that point.”
Human trials are expected to begin next month in the U.S., China, and South Korea. If the vaccine is found to be effective, the company has committed to producing 1 million doses of it before the end of 2020.
Last month, the Gates Foundation announced it would provide up to $100 million to improve detection, isolation and treatment efforts and and accelerate the development of vaccines, drugs and diagnostics.
Immunologist and physician Lynda Stuart, directs the foundation’s vaccine research, said we may need an approach that can get millions and even billions of doses.
Evidently, the approval process for vaccines faced higher thresholds for passing than for most medicines. Even if the samples pass clinical tests without a hitch, production of a usable coronavirus vaccine will probably take 12 to 18 months.
The Food and Drug Administration approves and licenses a vaccine before public use. Vaccines are developed in laboratories and tested on animals before clinical trials with human volunteers.
Tests are conducted in phases. In preclinical tests, the vaccine is tested in animals to measure toxicity, efficiency of dosage and methods to administer it, including oral, injection, intranasal and others.
At the Novavax Lab in Gaithersburg, Maryland in the United States, Dr Nita Patel is leading a team of all-female scientists are in phase two of development to create a viable vaccine for COVID-19. which means they’re being tested on animals. If they get that right, there may be two to three phases of human trials.
The team of more than 150 scientists are working day and night to isolate the virus and find a breakthrough vaccine against Covid-19 using recombinant nanoparticle technology.
Dr Patel is director of vaccine development at Novavax.
Females at the Forefront —
Meet the all #women team of #scientists looking to create the first viable #vaccine for #COVID19.
I got to see the @NovavaxInc lab in Gaithersburg, MD where they have three vaccine options they’re currently testing!@ABC7News #coronavirus #science pic.twitter.com/vtQpNICtjC— Victoria Sanchez (@VictoriaSanchez) February 28, 2020
Female scientists are also leading efforts to confront coronavirus.
At Johns Hopkins University, civil engineering professor Lauren Gardner has led a team to build a map based on information collected from various sources in China, the US and elsewhere to track the spread of the virus and locate areas where the virus is taking hold in real time and where it may attack more in future.
Gardner is the co-director of the Johns Hopkins Center for Systems Science and Engineering, led the team that designed a dashboard, which has a wide range of always-updating data, from the number of confirmed cases, the number of deaths from the virus, the number of people who have recovered, and cases by region and country.
Gardner said in a special briefing that statistics about users of the dashboard shows that the public is mostly using it “looking for reliable, factual information.”
Gardner said local, state and federal governments, public health agencies are also using it.
“In terms of [which countries are] using this dashboard, as far I can tell it’s pretty much everybody,” Gardner said. “It’s gone viral on every social media channel that exists. … I think that this really speaks to this huge demand for reliable, trustworthy, objective information, especially around situations like these.”
Statistics show people in the United States account for the majority of users. Gardner said her team has been taken aback but just how popular the dashboard has become.
“It’s been pretty popular for awhile,” Gardner said. “At the moment we’re getting well over a billion requests per day — or interactions with this dashboard on a daily basis.”
“It’s gone viral on almost every social media channel that exists. I think that this really speaks to this huge demand for reliable, trustworthy, objective information especially around situations like these,” Gardner said.
Earlier this week on Capitol Hill, Lauren Sauer, director of operations for the Johns Hopkins Office of Critical Event Preparedness and Response led a panel discussion of experts who shared the latest insights and evidence about how the COVID-19 virus is transmitted, how it is being tracked, and how governments, institutions, and individuals can prevent its spread.
Lisa Maragakis, was also on the panel. As the senior director of infection prevention for the Johns Hopkins Health System and an epidemiologist, Dr Maragakis told audiences that “testing capacity is not currently adequate and we need more.”
“We need this as soon as we can have it,” she said.
Officials at the World Health Organization said Monday that of about 80,000 people who have been sickened by COVID-19 in China, more than 70% have recovered and been discharged from hospitals.
World Health Organization’s Chief of Emergencies Dr. Mike Ryan said it can take up to six weeks for people to fully recover from COVID-19 infections.
Across the world, several biotech companies, universities, government agencies and organisations are teaming up to create a vaccine or treatment.